Upstream
VOGELBUSCH BIOPHARMA designs and supplies plants and systems for upstream processes, from the preparation, storage and filling of media to fermentation and harvesting. In any scenario, we can support and advise your company and plan the best possible solution for you:
Media preparation
Media preparation tanks including downstream sterilization and/or media filtration (depth filtration, bioburden reduction using 0.2 µm filters). The filtered media can be used directly in the fermentation process, or it is filled into media storage tanks or disposable bags and stored until further use.
We plan your media preparation tanks according to specific mixing time by using mixing simulations.
Typical features of filtration skids
- Modular construction suitable for various liquid filtration applications
- Flexible construction enables applications with single or multiple filter arrays and different filter configurations
- Filter lines with
- Pre- and main filter-units for bacterial count reduction
- Simple adaption mechanisms enable the use of a variety of filter housings (single – or multi-element housing with variable heights)
- Skids and all components are suitable for CIP (cleaning in place) and SIP (sterilization/sanitization in place)
- Optimized skid structure
- Rinse- and product-recovery steps (connections for buffer and high-purity water)
- Separate SIP for filter groups (pre-filter and main-filter)
- Filter integrity test of membrane filters
- Monitoring of inlet- and differential-pressure
- Monitoring and controlling of flow rate and volume
- Compatible with all common process control systems (PCS)
Liquid filtration-steps are standard methods of modern biotechnology.

Fermentation
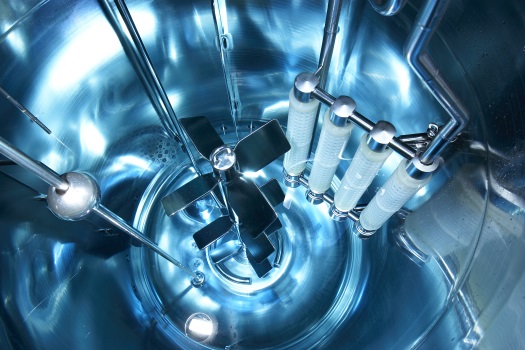
Bioreactors for microbial fermentation, propagation of cell cultures in suspension and cultivation of adherent cells on micro carriers
Fermentations techniques:
- Microbial fermentation – Batch, Fed-Batch
- Cell cultures – batch, fed-batch, continuous using perfusion (harvesting of cell-free culture supernatants), dialysis fermenter (removal of growth/product-inhibiting by-products from fermentation medium)
Harvesting
- Microbial fermentation: Production of pharmaceutical agents containing recombinant microorganisms, production of enzymes and hormones
Cell disruption by means of high-pressure homogenizers (processes using microbial fermentation) - Cell culture: Manufacture of pharmaceutical agents (enzymes and hormones), production of monoclonal antibodies for therapeutic and diagnostic applications, production of virus vaccines
Cell separation by means of filtration to obtain biomass or cell-free culture supernatant, batch-wise cell separation or continuous cell separation during fermentation by combining depth and 0.2µm membrane filtration.
Disposable technology
The use of disposable technology (single use) is often discussed as an alternative to stainless steel. This technology and it’s useful application offers the possibility to save time and costs during the qualification process and to manufacture a GMP-compliant product efficiently. For large-scale production, a combination of stainless steel systems and disposables is often suitable according to process steps and requirements.
The appropriate handling within the production area as well as the disposal of plastic materials must also be taken into account when using single-use technology.
We plan your application with disposable technology for the entire process or in combination with fixed stainless steel systems.
This can be media preparation and storage in stainless steel, cultivation of cell culture in the single use system up to 1,000 L and the production reactor (stainless steel) with 10,000 L.
In any case, we advise you individually and make sure to plan the best possible solution for you.